MedPage On the present time) — The FDA despatched 18 warning letters to web sites that illegally market unapproved and misbranded botulinum toxin (Botox) products, the company announced.
The letters had been addressed to sites with names fancy cosmo-korea.com, derma-resolution…
Study More
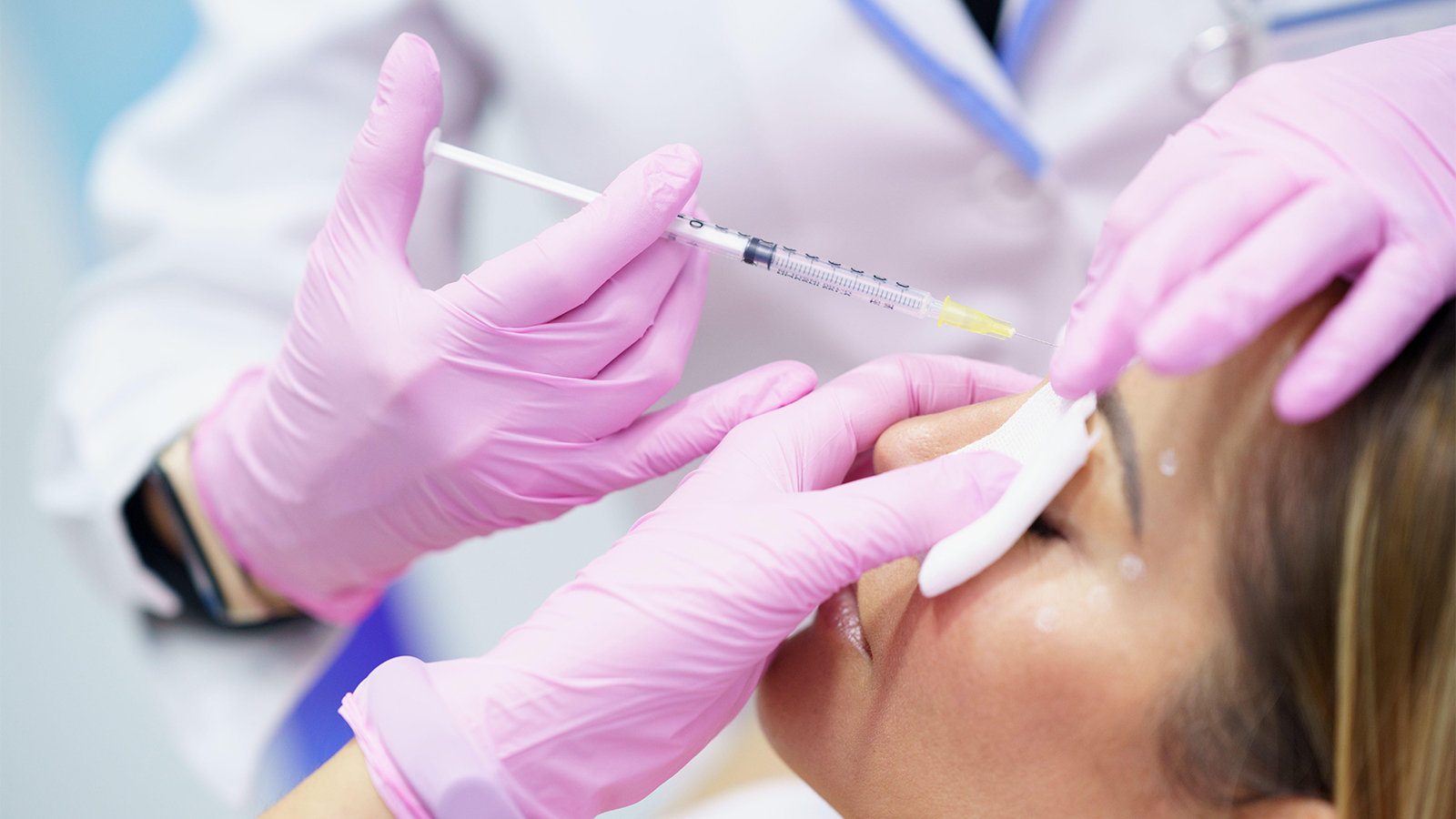
FDA’s Botox Warning Targets Web sites With Unapproved Products
You May Also Like
More From Author

Recentering ingenious and relevance in a precision-pushed industry








